9/27/2021
1,8-Diazabicyclo[5.4.0]undec-7-ene, or DBU for short, as a recognized sterically forbidden non-nucleophilic base, has been widely used in organic synthesis. In recent years, it has been found that DBU can also participate in the reaction as an effective nucleophilic base.
Introduction to 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)
1,8-Diazabicyclo[5.4.0]undec-7-ene, referred to as DBU, is an amidine with a heterocyclic structure. Its English name is 1,8-Diazabicyclo[5.4.0]undec-7 -ene, the molecular formula is C9H12N2, the structural formula is as shown in Figure 1, the molecular weight is 152.24, the CAS number is: 6674-22-2, it is a colorless or light yellow liquid at room temperature, soluble in water, ethanol, acetone and other organic solvents, generally stored below 30°C.

Formula 1
DBU has been widely used in organic synthesis as a recognized sterically forbidden non-nucleophilic base, especially in dehydrohalogenation reactions. For example, under the action of DBU, N-halogenated amides can be successfully dehydrohalogenated and rearranged to obtain isocyanate (Formula 2).

Formula 2
In recent years, research findings have shown that: DBU can also participate in the reaction as an effective nucleophilic base. DBU itself will undergo nucleophilic attack with the α, β unsaturated system to form ε-caprolactam derivatives. In addition, as a nucleophilic base, DBU can participate in many reactions. like:
1. In the Baylis-Hillman reaction, DBU has higher catalytic activity than other amine catalysts because it has better stability in the reaction and can accelerate the reaction (Formula 3).

Formula 3
2. In the conversion of primary or secondary nitroalkanes to aldehydes or ketones, the DBU/acetonitrile system exhibited very specific inductive activity compared with the original strong acid system. can convert secondary nitro compounds to ketones in high yield, high chemo- and regioselectivity under homogeneous basic conditions in the presence of primary nitro compounds (Eq. 4).

Formula 4
3. Under the induction or catalysis of DBU, a new carbon dioxide chemical fixation method was developed. Carbon dioxide can react mildly with 2-aminobenzonitrile to generate drug intermediate quinazoline derivatives (Formula 5).

Formula 5
4. As a nucleophilic catalyst, DBU can use non-toxic methylation and benzylation reagents (such as dimethyl carbonate or dibenzyl carbonate) to achieve nucleophilic substitution of substrates containing N, O, and S atoms reaction. provides an environmentally friendly method for the methylation or benzylation of heteroatom-containing substrates (Eq. 6).
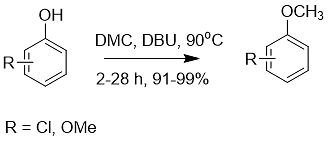
Formula 6
5. DBU can also mildly realize the cleavage reaction of the N-acetyl or N-phenyl groups of carbazole, indole, and nitroaniline substrates (Formula 7). The reaction can also be carried out in acetonitrile or under microwave irradiation.
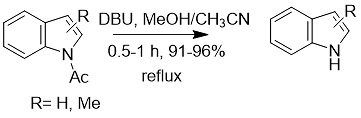
Formula 7
6. DBU has shown an important use in drug synthesis. For example: the spirocyclization reaction of the substrate is realized in anhydrous acetonitrile (Formula 8).
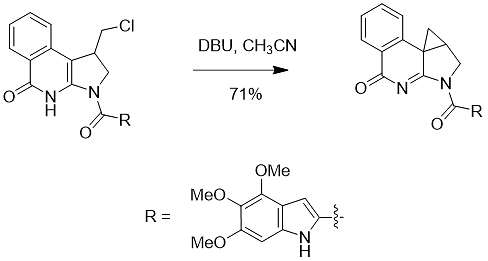
Formula 8
Under the catalysis of DBU, aldehydes, malononitrile, and 4-hydroxycoumarin were reacted to synthesize chromene derivatives (Eq. 9).
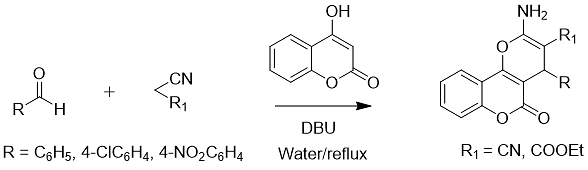
Formula 9
This article briefly introduces some applications of DBU in organic synthesis. It is widely used and has the characteristics of mild reaction conditions, simplified synthesis steps, specific product selectivity, and high yield.
Haofan Biological Co., Ltd. is committed to the research development and production of amide and peptide synthesis reagents. After more than ten years of development and accumulation, the company has become the world's largest and most complete supplier of amide synthesis reagents. Welcome friends to inquire.
Please fill out the form below and our sales team will be happy to assist you with a quote on peptide synthesis reagents.